This month's highlight

Newsletter
November 2024
One Source Partner for Genome Editing: Introducing cGMP mRNA
BioSpring has been granted manufacturing authorization (ICH Q7).jpg?width=614&height=409&name=2024_sept_25_biospring_reaktor_SWF_1852%20(1).jpg) for cGMP-grade mRNA production from the German authorities, which confirms that we fulfill the cGMP quality standards for mRNA production by in vitro transcription (IVT). After 17 years of successfully producing cGMP oligonucleotides for clinical and commercial applications, we are thrilled to announce the addition of cGMP mRNA to our offerings.
for cGMP-grade mRNA production from the German authorities, which confirms that we fulfill the cGMP quality standards for mRNA production by in vitro transcription (IVT). After 17 years of successfully producing cGMP oligonucleotides for clinical and commercial applications, we are thrilled to announce the addition of cGMP mRNA to our offerings.
"With the addition of cGMP mRNA to our manufacturing portfolio, we are now taking the decisive step towards becoming the world's leading supplier of cGMP RNA for genome editing applications.", remarks Dr. Sylvia Wojczewski, CEO of BioSpring. "We can now offer our clients cGMP mRNA and cGMP guide RNA for therapeutic applications from a single source."
We are pleased to share that our cGMP production capacities for mRNA and guide RNA have significantly expanded, thanks to our additional, newly constructed facilities in Frankfurt, Germany. This enhancement will strengthen our commitment to supporting genome editing applications and make us one of the world leaders in the production of genome editing components. The newest production site features additional state-of-the-art cleanrooms for efficient and simultaneous production of cGMP guide RNA and mRNA in custom large and small batch sizes. Moreover, with this expansion, we tripled our production capacity for guide RNA, which now enables an annual throughput of batches in the triple digit range. We now have the world’s largest cGMP guide RNA production capacities for genome editing programs.
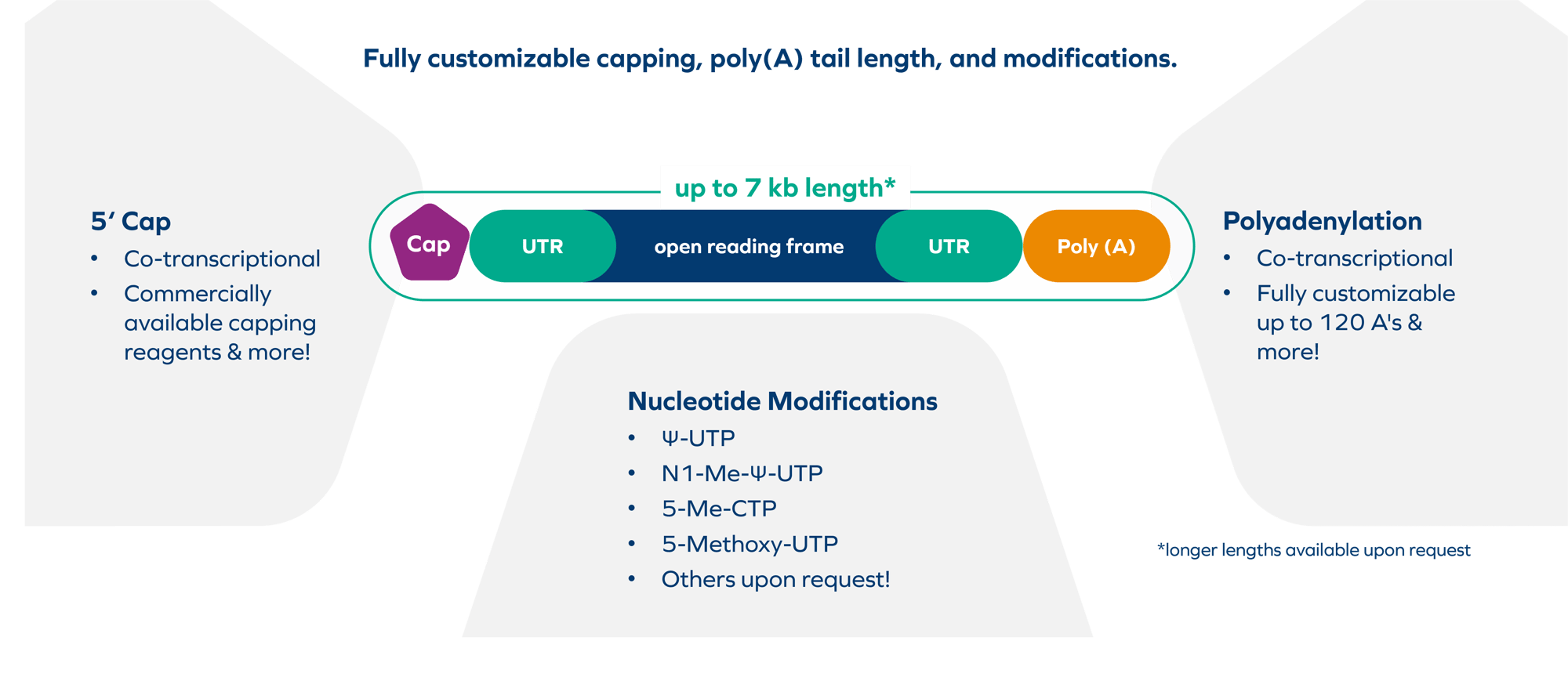
Scalable sizes from micrograms up to 250 grams per batch are now possible for the production of mRNA under cGMP conditions. Through BioSpring's innovative production technologies, we can offer our products and services without the need of paying patent/license fees. With more than 20 years of producing RNA modalities at BioSpring, we can leverage our extensive knowledge in purification as well as release analysis to yield highly pure and stable product. Our advanced purification technology enables the production of cGMP RNA of the highest purity and quality. “We offer end-to-end services for custom IVT mRNA production and accommodate lengths up to seven kilobases or higher”, remarks Dr. Marcus Merkel, Head of mRNA Production at BioSpring.
We also provide extensive in-house analytical development and testing, including method validation, characterization, release testing, and stability studies for mRNA. The high-fidelity analytics of BioSpring utilize cutting-edge techniques such as NGS (next-generation sequencing) and HRMS (high-resolution mass spectrometry). With a dedicated quality team, we take rigorous steps to ensure the quality of your mRNA.
This certification reinforces our commitment to being a leading global provider of RNA for genome editing applications. We’re excited to continue supporting innovation in this vital field.
Learn more about our mRNA services.
This Month's service in focus
Unlock the Full Potential of Your mRNA Therapeutics with BioSpring's Cutting-Edge Analytical Solutions
At BioSpring, we understand that the success of your mRNA programs hinges on precision and quality. That’s why we offer analytical solutions designed to optimize the stability and integrity of your mRNA products across every stage of development.
Our suite of services includes:
- High-Resolution Analysis: Ensure the integrity of your mRNA with single analysis, high-resolution mass spectrometry (HRMS) for capping and poly(A) tail evaluation, and ELISA for detecting double-stranded RNA (dsRNA).
- Advanced Sequencing: Leverage the power of next-generation sequencing (NGS) and MS/MS for highly detailed molecular analysis, ensuring your product's fidelity.
- Precision dsRNA Quantification: With ultra-sensitive dsRNA detection methods, we ensure precise measurements to maintain the highest product quality.
- Alternative RNA Quantification: Tailored approaches to meet your unique project needs, ensuring accurate ribonucleic acid quantification.
R&D-Grade Release Testing Scope Includes:
- Integrity by capillary electrophoresis (CE)
- Purity and impurity analysis
- UV absorbance and mRNA concentration
- Optional endotoxin testing
BioSpring's expert team is ready to advance your mRNA therapeutics from research to market. Let us be your trusted partner in driving innovation and ensuring the quality you need for success.
Interested in learning more? Take the next step in your mRNA therapeutic journey.
Contact us
Related Research insights
Elevating guide RNA Quality Control through In Vitro Activity Assay
At this year’s Bioassays conference, Dr. Christian Sattler, QC Manager Activity Assay, unveiled research on the development of an innovative in vitro guide RNA activity assay. His poster highlighted a significant advancement in quality control for guide RNA, particularly for therapeutic applications involving CRISPR/Cas9 technologies. The new assay offers a precise, reliable method to evaluate the activity of guide RNA in a regulated environment.
Benefits for our clients:
- Comprehensive Activity Analysis: This assay provides our clients detailed insights into guide RNA functionality, including cutting efficiency and EC50 values. Such data are essential for clients involved in genome editing and therapeutic development, offering a clear understanding of guide RNA performance.
- Batch Comparability and Modification Impact: The assay allows for the comparison of different guide RNA batches and evaluates the effects of various modifications. This capability supports clients in optimizing guide RNA designs for specific applications, enhancing therapeutic outcomes.
- Versatility for Advanced Applications: Beyond standard quality control, the assay's versatility enables new research opportunities, such as target screening, protein activity studies, and kinetic analyses using molecular beacons.
- Real-Time Kinetic Data: The inclusion of molecular beacons provides real-time data on RNP (ribonucleoprotein complex) reactions, allowing for advanced kinetic studies and deeper insights into guide RNA-mediated DNA cleavage mechanisms.
The Role of the In Vitro Assay in guide RNA Quality Control
Ensuring the quality and functionality of guide RNA is critical in the development of CRISPR-based therapies. The guide RNA must effectively interact with the Cas9 enzyme to mediate precise DNA cleavage, a key step in gene editing. The newly developed assay at BioSpring evaluates this activity in vitro by using plasmid target DNA and capillary electrophoresis analysis. This method allows for the accurate measurement of DNA cleavage efficiency and provides crucial data on the potency and effectiveness of guide RNA batches.
How the Assay Works
The guide RNA activity assay involves several key steps to ensure accurate and reliable results:
- Target DNA Preparation: The DNA sequence containing the guide RNA recognition site is cloned into a plasmid, which is then linearized using a restriction enzyme. This prepares the DNA for interaction with the guide RNA-Cas9 complex.
- Activity Assay Execution: The target DNA and guide RNA are subjected to controlled heating and cooling to resolve secondary structures. Cas9 is then added to form the guide RNA-Cas9 complex (RNP). This complex is incubated with the target DNA, enabling Cas9 to cleave the DNA at specific sites, resulting in two fragments.
- Capillary Electrophoresis Analysis: The resulting DNA fragments are analyzed using capillary electrophoresis, which provides a detailed profile of the cleavage products. This analysis is used to calculate the cutting efficiency and determine the EC50 value for each guide RNA.
Empowering Clients with Reliable Data
The in vitro guide RNA activity assay developed by BioSpring offers clients a robust tool for ensuring the highest standards in guide RNA quality control. By providing precise, reliable data on guide RNA activity, clients can confidently develop and optimize their gene-editing technologies. This assay also facilitates the exploration of new therapeutic avenues and enhances the understanding of guide RNA modifications, supporting the development of next-generation CRISPR therapies.
Continued Advancements
The insights presented by Dr. Sattler highlight the potential of this new assay in guide RNA research and therapeutic applications. The ability to obtain real-time kinetic data and perform comprehensive activity analyses opens new possibilities for therapeutic development and molecular research. As BioSpring continues to innovate, we remain committed to providing our clients with the most advanced tools and technologies to drive their research forward.
Get to know our experts
Sarah Schäfer
QC Client Project Specialist
Sarah studied Chemistry and Biochemistry at the Ludwig Maximilians University in Munich, Germany. In 2019, she joined the Quality Control (QC) department at BioSpring, where she has been working as Client Project Specialist since 2023, providing QC support for our clients’ projects.
What do you like about your work?
"What I like most is the variety in my day-to-day work. Working closely with the specialized project teams and clients, you can grow both personally and in the role of QC project SME. I also enjoy the proximity to our analytical laboratory and the fact that I can analytically accompany the development in the advancing field of oligonucleotides."
How have your studies prepared you for working at BioSpring?
"Participating in the many laboratory practicals and getting to know the various instruments and methods are crucial for my daily work. In addition to the specialist knowledge acquired, the experience of working in international university groups and the independent planning and organization of projects are a good basis for working at BioSpring."
What are your daily challenges as a QC client project specialist?
"The wide range of different oligonucleotides and the fact that each molecule has different properties are definitely the biggest challenges in analytics. In addition, you have to adapt to the constantly changing regulatory requirements and analytical possibilities."
Can you share a memorable project or accomplishment during your time here?
"My first project, which I supported from the beginning of process development to the final cGMP release of the first batch, is always something to remember. "
What do you like to do in your free time?
"In the summer season, I prefer to spend my time outdoors by the lake or sea or hiking in the mountains. Otherwise, I enjoy spending time with family and friends over a convivial evening."
In case you missed it
Recent BioSpring News
BioSpring Knowledge Base
 We recently introduced our new Knowledge Base on our website, your place to explore our impact, expertise, and services all in one place. Take a look at the areas of research and clinical studies BioSpring services have supported in our Oligos in Action section, explore our academic contributions in Our Publications and Posters, or learn more about our products and services through our Flyers & Brochures.
We recently introduced our new Knowledge Base on our website, your place to explore our impact, expertise, and services all in one place. Take a look at the areas of research and clinical studies BioSpring services have supported in our Oligos in Action section, explore our academic contributions in Our Publications and Posters, or learn more about our products and services through our Flyers & Brochures.
Where to find us next

